Research at Stanford has resulted in a rechargeable "battery" that uses a mix of sea and fresh water and a dash of nanotech to generate electricity. Green power plants at river mouths could be the ultimate result.
By radically tweaking the way saltwater/freshwater batteries work, a research team led by professor Yi Cui has put together a new technology that could supply about 2 terawatts of electricity annually--that's about 13% of the world's current energy needs. The unlikely scenario behind this figure would require every single one of the world's river's mouths to be converted into saltwater power-generation systems. But even at a smaller scale, the technology could result in useful clean power-generation systems anywhere you can bring salt and freshwater together.
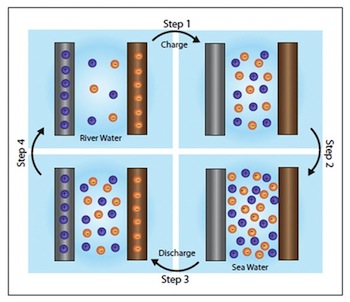 The technology works through an incredibly simple process (though it requires a complex explanation). Two electrodes are immersed in a liquid bath that contains freshwater. A very small electric current is applied across the electrodes to charge up the "battery," then the freshwater is drained to be replaced by seawater. The seawater's greater concentration of ions from the dissolved salt (60 to 100 times more, in fact) results in an increase to the voltage across the two electrodes--meaning the battery can then be discharged to extract more energy than was initially applied to charge it.
The technology works through an incredibly simple process (though it requires a complex explanation). Two electrodes are immersed in a liquid bath that contains freshwater. A very small electric current is applied across the electrodes to charge up the "battery," then the freshwater is drained to be replaced by seawater. The seawater's greater concentration of ions from the dissolved salt (60 to 100 times more, in fact) results in an increase to the voltage across the two electrodes--meaning the battery can then be discharged to extract more energy than was initially applied to charge it.
Batteries that utilize this technology already exist, but they can be fragile and typically only rely on one type of ion migration. The Stanford system is much more resilient and also uses both the sodium ions and chlorine ions from the salt in the seawater to generate power--making it more efficient. Greater efficiency still is achieved by building the positive electrode out of manganese dioxide nanorods, which hugely increase the surface area of the electrode (by around 100 times compared to traditional electrode materials).
The idea for power plant production would be to locate huge versions of these batteries where seawater and freshwater are readily available. One could protect sensitive coastal areas from the impact of the plants by using a proportion of the electricity produced to pump seawater inland. Or instead of using river water, a different source of freshwater would suffice--rain run-off, gray water byproducts from industry, and possibly even treated sewage.
Does it all sound too good to be true? Well, it gets better: A supply of just 50 cubic meters of freshwater per second could produce up to 100 MW of power, enough to power 100,000 homes. And the waste byproduct is merely a mix of sea- and freshwater, easily pumped into the ocean. Even the nanotech coating on the electrodes is environmentally benign.
Image via Flickr user Sbeebe.
To read more news like this follow Fast Company on Twitter. Or chat with Kit Eaton himself via tweets.
Related: How Resource-Strained Cities Can Save Water
T.A.T.u. Amber Valletta Paris Hilton Victoria Pratt Shakara Ledard
No comments:
Post a Comment